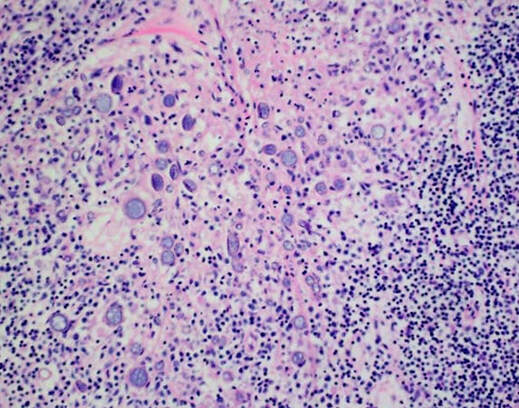
 Coccidiodes immitis spherules in tissue (aka valley fever), lymphohistiocytic inflammatory response
Coccidiodes immitis spherules in tissue (aka valley fever), lymphohistiocytic inflammatory response
In the descriptions of specific areas of study below, the time spent in each area is for a one year fellowship program. For Ph.D. or M.D. candidates without significant relevant clinical experience (for example, M.D. with training outside of infectious diseases or clinical pathology) core training will be expanded in the context of a two year program to increase proportionate exposure in subspecialty areas, allow time for further development of clinical microbiology research projects, and gain greater exposure to clinical infectious diseases issues through rounding with the ID team for extended intervals. In a two year program, exposure in these areas will be proportionally increased and will emphasize acquisition of adequate expertise in medical and public health microbiology to direct a clinical microbiology laboratory. The following is a summary of training goals:
Bacteriology and Antimicrobial Susceptibility Testing (3 months): Fellows will learn identification methods (by rotating through blood, urine, respiratory, genital, stool, and wound benches) and susceptibility testing methods. Instruction will be through observation, review of all procedure manuals, and progression to independence on a simpler bench such as the urine bench with review by one of our lead technologists. Fellows will progress to independent reading of gram stains. Fellows will learn how to plant specimens and spend time helping in the intake area. Fellows will become intimately familiar with CLSI standards for the bacteriology laboratory susceptibility testing of aerobic, anaerobic, and fastidious organisms and work with lead technologists to ensure that yearly updates are promptly incorporated into laboratory procedures. It should be emphasized that we do not view the fellow as a replacement for our microbiology technologists but rather believe philosophically that fellows should attain some degree of familiarity with day to day activities so that they may better trouble-shoot and understand the functioning of the laboratory in their future role as a laboratory director. We also view the “planting” area as a critical step in the pre-analytic integrity of our data. Fellows will learn how to plant specimens and spend time helping in the intake area.
Fellows will round with the microbiology attendings and residents during the afternoons and will help trouble-shoot cases. They will review critical results during the daytime (for example, positive CSF Gram stains and positive blood cultures) in conjunction with clinical history to determine whether proactive intervention (communication, further testing, alternative antimicrobial therapy) will contribute positively to patient care and, if so, act accordingly.
Mycology, Mycobacteriology, and Parasitology (1 month each): Fellows will learn how to identify fungi and yeast including the preparation of tease and scotch tape preps. In mycobacteriology, they will learn the decontamination process, the specifics of safe processing of these specimens, and the performance of Kinyoun and fluorochrome staining. They will become proficient in reading these stains through review of all positives. They will learn to process parasitology specimens (wet preps, concentrates, and permanent smears). They will learn how to identify parasites through review of present and past material, the use of study sets, participating in the CDC parasitology mailing list, and review of past and present parasitemia blood smears.
They will provide initial diagnosis of all positive parasitemia smears on an on-call basis. They will take and pass an initial blood parasite speciation competency examine within the first month of their training, interpret malaria smears, and may participate in CAP proficiency testing determination for blood-borne parasite speciation on a rotational basis with attendings.
Virology, Mycoplasma, Ureaplasma, Chlamydia (1 month): Fellows will learn basic aspects of tissue culture (if not already familiar). They will learn how to identify CPE, how to perform shell vial staining, and how to perform DFA staining in rapid respiratory viral panels. They will prepare demonstrations of CPE for plate rounds presentations.
During the month spent at Children’s Hospital Boston, fellows will spend two weeks in the full-service virology laboratory. They will learn the advantages and disadvantages of the several different methods for diagnosis of respiratory viruses (conventional culture, shell vial culture and immunofluorescence) as well as the use of quantitative real-time PCR (RT-PCR) for detection of herpes simplex virus and Epstein-Barr virus. Additional RT- PCR assays will be validated over the next few years, and so fellows will be exposed to new assay development and validation.
Morphologic evaluation of microbiological agents identified in fluids removed from patients (bronchoalveolar lavage, direct wound smears) and in tissue specimens (autopsy and surgical pathology) – ongoing. Fellows will develop competence in reading primary Gram stains from direct patient specimens and will be available for consultation by technologists for interpretation of difficult specimens, which may also require interpretation in the context of clinical history. Fellows will learn how to prepare cytospin Gram stains. They will learn how to perform and interpret various techniques for examination of primary specimens, also including KOH/calcofluor detection of fungal forms, direct fluorescence antigen detection of PCP in BAL and induced sputum specimens, and acid fast and modified acid fast stains for Mycobacteria and Nocardia. Fellows will perform STAT intraoperative Gram stains on the BIDMC West Campus on a rotational basis with residents, participate in the CAP Gram stain survey, and assist in teaching of pathology residents in Gram stain interpretation.
They will become familiar with the range of morphologies of clinically-relevant organisms in stains of primary specimens vs. cultured organisms, and of organisms in histopathological sections, through the use of computer programs, teaching sets, and review of relevant books in our library. The microbiology service reviews all non-obvious diagnoses from the anatomic pathology service, usually related to the diagnosis of filamentous fungal, dimorphic fungal, yeast, and mycobacterial infections in histological sections. They will learn the use of special stains in identification of specific organisms (acid fast, GMS, Fontana-Mason, Mucicarmine, PAS, Brown and Brenn, Dieterle, etc.) and become familiar with these organisms' appearance when stained with these methods. Along with the attending the fellow will prepare a description of our findings for use in the final histopathological report. The application of immunohistochemical and immunofluorescent techniques performed in our own laboratory or elsewhere (e.g., Legionella DFA) will also be taught.
Fellows along with our residents serve as a direct consultative liaison with the infectious diseases service. They will review primary Gram stains of cases of particular medical significance (CSF and other sterile body fluids), investigate their clinical context (for example, determining whether organisms are likely to be a true infection vs. a contaminant through examination of WBC and protein count). Based on the primary staining qualities, they will develop a differential diagnosis to aid in the institution of the most appropriate therapy (e.g. potential Listeria vs. Enterobacteriaceae).
Molecular Diagnostics (1 months): Fellows will spend approximately one month learning the molecular diagnostic technologies both at our institutional laboratories. At present BIDMC performs viral load testing for HIV, HBV, HCV, and CMV quantitiative viral load testing; and GC/Chlamydia using real time PCR and transcription-mediated amplification technologies. SARS-CoV-2 nucleic acid amplification testing is performed on several platforms: Abbott M2000, Abbott alinity m, Cepheid, GenMark ePlex and Abbott ID Now. C. diff, norovirus, and Staph aureus screening is performed using Cepheid random access technology. Fellows will learn all aspects of different molecular assays, from nucleic acid extraction to quality control procedures. Through directed independent study they will learn about the major molecular diagnostics methods employed in clinical microbiology. The fellow will become familiar with automated extraction methodology. The molecular diagnostics laboratory is in the process of change with intent to bring in a number of ASR and new commercial methods over the next few years. Fellows will directly participate in the evaluation and implementation of new assays. We perform pulsed-field gel electropheresis for outbreak investigation, and use Minion next generation sequencing technology for investigation of novel Gram-negative pathogen resistance. Fellows will become intimately familiar with CLSI recommendations for verification, validation, and other quality assurance measures in molecular diagnostics and will ensure that they are being applied consistently in the laboratory. Note, the state laboratory rotation and Children’s hospital rotations also include learning about an extensive array of additional molecular diagnostic assays and platforms including next generation sequencing. Taken together with platforms at BIDMC, these include representatives of almost all commercial, analyte specific agent, and home brew approaches used in modern molecular diagnostics laboratories.
Infectious Disease Serology Testing (Approximately 0.5 months): Fellows will become familiar with serological testing methods.
Daily Rounds (ongoing): Fellows will round with the microbiology attendings and residents during the afternoons and will help trouble-shoot cases. They will review critical results during the daytime (for example, positive CSF gram stains and positive blood cultures) in conjunction with clinical history to determine whether proactive intervention (communication, further testing, alternative antimicrobial therapy) will contribute positively to patient care and, if so, act accordingly. We also review consults from anatomic pathology to help define and identify infectious disease processes in histology specimens.
Pediatric Microbiology: Children's Hospital Boston (1 month rotation): This four week rotation will round out fellows' training by exposure to organism subsets and clinical issues that are specific to the care of children, as described earlier in this application. For example, Children’s Hospital is a major referral center for Cystic Fibrosis and uses special techniques to identify Burkholderia and other pathogens infecting this population, and has a robust virology laboratory related to childhood viral infections which includes molecular diagnostics using newer platforms.
Public Health Microbiology (1 month): A four to five week rotation at Massachusetts State Laboratory Institute will expose fellows to public health microbiology and familiarize them with diagnostic techniques unique to that setting. Laboratory rotations will include the mycobacteriology laboratory (where susceptibility testing and identification procedures not performed at BIDMC will be learned), enteric lab (where they will be introduced to strain typing and associated outbreak surveillance methodology), molecular diagnostics, and specialized virology laboratories. The laboratory has an extensive molecular diagnostics program related to arbovirus surveillance and diagnosis of emerging infectious diseases. Fellows in the past for have gone on field rips to learn how to capture mosquitoes for arbovirus surveillance efforts. In addition, fellows will become familiar with the state laboratory’s role in the laboratory response network.
Bacteriology and Antimicrobial Susceptibility Testing (3 months): Fellows will learn identification methods (by rotating through blood, urine, respiratory, genital, stool, and wound benches) and susceptibility testing methods. Instruction will be through observation, review of all procedure manuals, and progression to independence on a simpler bench such as the urine bench with review by one of our lead technologists. Fellows will progress to independent reading of gram stains. Fellows will learn how to plant specimens and spend time helping in the intake area. Fellows will become intimately familiar with CLSI standards for the bacteriology laboratory susceptibility testing of aerobic, anaerobic, and fastidious organisms and work with lead technologists to ensure that yearly updates are promptly incorporated into laboratory procedures. It should be emphasized that we do not view the fellow as a replacement for our microbiology technologists but rather believe philosophically that fellows should attain some degree of familiarity with day to day activities so that they may better trouble-shoot and understand the functioning of the laboratory in their future role as a laboratory director. We also view the “planting” area as a critical step in the pre-analytic integrity of our data. Fellows will learn how to plant specimens and spend time helping in the intake area.
Fellows will round with the microbiology attendings and residents during the afternoons and will help trouble-shoot cases. They will review critical results during the daytime (for example, positive CSF Gram stains and positive blood cultures) in conjunction with clinical history to determine whether proactive intervention (communication, further testing, alternative antimicrobial therapy) will contribute positively to patient care and, if so, act accordingly.
Mycology, Mycobacteriology, and Parasitology (1 month each): Fellows will learn how to identify fungi and yeast including the preparation of tease and scotch tape preps. In mycobacteriology, they will learn the decontamination process, the specifics of safe processing of these specimens, and the performance of Kinyoun and fluorochrome staining. They will become proficient in reading these stains through review of all positives. They will learn to process parasitology specimens (wet preps, concentrates, and permanent smears). They will learn how to identify parasites through review of present and past material, the use of study sets, participating in the CDC parasitology mailing list, and review of past and present parasitemia blood smears.
They will provide initial diagnosis of all positive parasitemia smears on an on-call basis. They will take and pass an initial blood parasite speciation competency examine within the first month of their training, interpret malaria smears, and may participate in CAP proficiency testing determination for blood-borne parasite speciation on a rotational basis with attendings.
Virology, Mycoplasma, Ureaplasma, Chlamydia (1 month): Fellows will learn basic aspects of tissue culture (if not already familiar). They will learn how to identify CPE, how to perform shell vial staining, and how to perform DFA staining in rapid respiratory viral panels. They will prepare demonstrations of CPE for plate rounds presentations.
During the month spent at Children’s Hospital Boston, fellows will spend two weeks in the full-service virology laboratory. They will learn the advantages and disadvantages of the several different methods for diagnosis of respiratory viruses (conventional culture, shell vial culture and immunofluorescence) as well as the use of quantitative real-time PCR (RT-PCR) for detection of herpes simplex virus and Epstein-Barr virus. Additional RT- PCR assays will be validated over the next few years, and so fellows will be exposed to new assay development and validation.
Morphologic evaluation of microbiological agents identified in fluids removed from patients (bronchoalveolar lavage, direct wound smears) and in tissue specimens (autopsy and surgical pathology) – ongoing. Fellows will develop competence in reading primary Gram stains from direct patient specimens and will be available for consultation by technologists for interpretation of difficult specimens, which may also require interpretation in the context of clinical history. Fellows will learn how to prepare cytospin Gram stains. They will learn how to perform and interpret various techniques for examination of primary specimens, also including KOH/calcofluor detection of fungal forms, direct fluorescence antigen detection of PCP in BAL and induced sputum specimens, and acid fast and modified acid fast stains for Mycobacteria and Nocardia. Fellows will perform STAT intraoperative Gram stains on the BIDMC West Campus on a rotational basis with residents, participate in the CAP Gram stain survey, and assist in teaching of pathology residents in Gram stain interpretation.
They will become familiar with the range of morphologies of clinically-relevant organisms in stains of primary specimens vs. cultured organisms, and of organisms in histopathological sections, through the use of computer programs, teaching sets, and review of relevant books in our library. The microbiology service reviews all non-obvious diagnoses from the anatomic pathology service, usually related to the diagnosis of filamentous fungal, dimorphic fungal, yeast, and mycobacterial infections in histological sections. They will learn the use of special stains in identification of specific organisms (acid fast, GMS, Fontana-Mason, Mucicarmine, PAS, Brown and Brenn, Dieterle, etc.) and become familiar with these organisms' appearance when stained with these methods. Along with the attending the fellow will prepare a description of our findings for use in the final histopathological report. The application of immunohistochemical and immunofluorescent techniques performed in our own laboratory or elsewhere (e.g., Legionella DFA) will also be taught.
Fellows along with our residents serve as a direct consultative liaison with the infectious diseases service. They will review primary Gram stains of cases of particular medical significance (CSF and other sterile body fluids), investigate their clinical context (for example, determining whether organisms are likely to be a true infection vs. a contaminant through examination of WBC and protein count). Based on the primary staining qualities, they will develop a differential diagnosis to aid in the institution of the most appropriate therapy (e.g. potential Listeria vs. Enterobacteriaceae).
Molecular Diagnostics (1 months): Fellows will spend approximately one month learning the molecular diagnostic technologies both at our institutional laboratories. At present BIDMC performs viral load testing for HIV, HBV, HCV, and CMV quantitiative viral load testing; and GC/Chlamydia using real time PCR and transcription-mediated amplification technologies. SARS-CoV-2 nucleic acid amplification testing is performed on several platforms: Abbott M2000, Abbott alinity m, Cepheid, GenMark ePlex and Abbott ID Now. C. diff, norovirus, and Staph aureus screening is performed using Cepheid random access technology. Fellows will learn all aspects of different molecular assays, from nucleic acid extraction to quality control procedures. Through directed independent study they will learn about the major molecular diagnostics methods employed in clinical microbiology. The fellow will become familiar with automated extraction methodology. The molecular diagnostics laboratory is in the process of change with intent to bring in a number of ASR and new commercial methods over the next few years. Fellows will directly participate in the evaluation and implementation of new assays. We perform pulsed-field gel electropheresis for outbreak investigation, and use Minion next generation sequencing technology for investigation of novel Gram-negative pathogen resistance. Fellows will become intimately familiar with CLSI recommendations for verification, validation, and other quality assurance measures in molecular diagnostics and will ensure that they are being applied consistently in the laboratory. Note, the state laboratory rotation and Children’s hospital rotations also include learning about an extensive array of additional molecular diagnostic assays and platforms including next generation sequencing. Taken together with platforms at BIDMC, these include representatives of almost all commercial, analyte specific agent, and home brew approaches used in modern molecular diagnostics laboratories.
Infectious Disease Serology Testing (Approximately 0.5 months): Fellows will become familiar with serological testing methods.
Daily Rounds (ongoing): Fellows will round with the microbiology attendings and residents during the afternoons and will help trouble-shoot cases. They will review critical results during the daytime (for example, positive CSF gram stains and positive blood cultures) in conjunction with clinical history to determine whether proactive intervention (communication, further testing, alternative antimicrobial therapy) will contribute positively to patient care and, if so, act accordingly. We also review consults from anatomic pathology to help define and identify infectious disease processes in histology specimens.
Pediatric Microbiology: Children's Hospital Boston (1 month rotation): This four week rotation will round out fellows' training by exposure to organism subsets and clinical issues that are specific to the care of children, as described earlier in this application. For example, Children’s Hospital is a major referral center for Cystic Fibrosis and uses special techniques to identify Burkholderia and other pathogens infecting this population, and has a robust virology laboratory related to childhood viral infections which includes molecular diagnostics using newer platforms.
Public Health Microbiology (1 month): A four to five week rotation at Massachusetts State Laboratory Institute will expose fellows to public health microbiology and familiarize them with diagnostic techniques unique to that setting. Laboratory rotations will include the mycobacteriology laboratory (where susceptibility testing and identification procedures not performed at BIDMC will be learned), enteric lab (where they will be introduced to strain typing and associated outbreak surveillance methodology), molecular diagnostics, and specialized virology laboratories. The laboratory has an extensive molecular diagnostics program related to arbovirus surveillance and diagnosis of emerging infectious diseases. Fellows in the past for have gone on field rips to learn how to capture mosquitoes for arbovirus surveillance efforts. In addition, fellows will become familiar with the state laboratory’s role in the laboratory response network.